Screenshot of a figure from the research article, DOI: 10.1016/j.device.2024.100517.
Researchers Develop An Implantable Sensor To Combat Opioid Overdoses
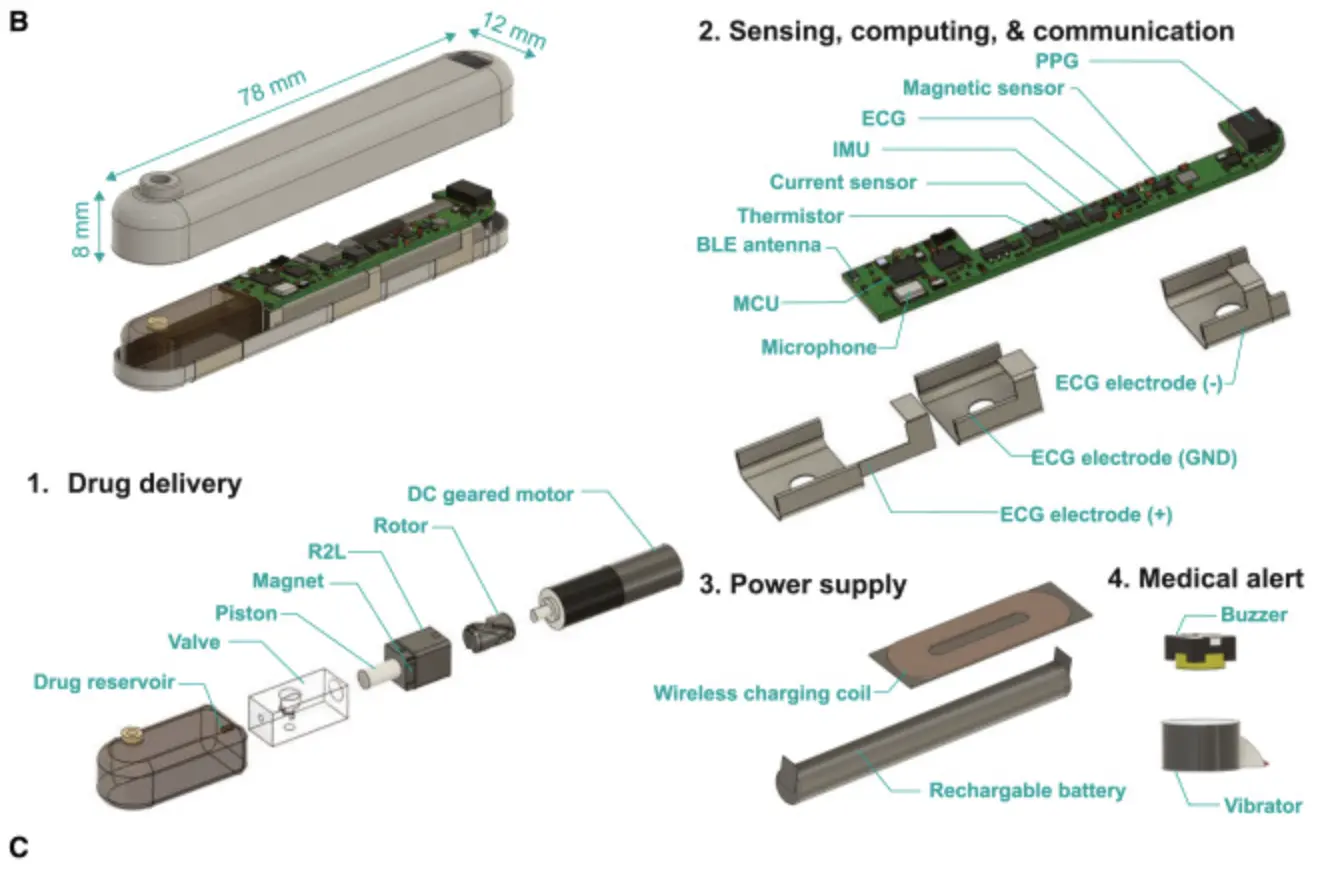
Researchers at MIT and Brigham and Women’s Hospital have developed an implantable device aimed at addressing the opioid overdose crisis. This small, gum-sized device, implanted under the skin, is designed to monitor vital signs and deliver naloxone, an opioid antidote, in the event of an overdose.
The study published on August 14 in the journal Device showcases the functionality of this new device. The implanted robotic device aims to overcome the limitations of current overdose reversal systems.
While naloxone is effective in reversing overdoses, the research paper reported that with current systems, less than 5% of overdose victims survive. This low survival rate is often due to delays or the absence of first responder intervention. Contributing factors include low user compliance with existing reversal systems, inaccurate overdose detection, and slow drug delivery.
To address these limitations, the researchers developed a subcutaneously implantable robotic first responder. The device continuously monitors vital signs, and employs an algorithm to detect opioid overdoses based on cardio-respiratory patterns. In case of an overdose, the device rapidly delivers a 10mg dose of naloxone in under 10 seconds.
The study trials demonstrated the system’s effectiveness in reviving 96% of overdosed pigs within 3.2 minutes. Additionally, the device was effective in distinguishing overdoses from other conditions, such as sleep apnea.
These results highlight the potential of this implantable technology to significantly improve survival rates.
“This could really address a significant unmet need in the population that suffers from substance abuse and opiate dependency to help mitigate overdoses, with the initial focus on the high-risk population,” said Giovanni Traverso, a senior author of the study, in an MIT News Announcement.
As reported in the MIT News Announcement, the researchers aim to further refine the device to enhance user comfort and integration. The next steps involve testing the device in human trials, which could commence within the next three to five years. They also plan to address the device’s miniaturization and battery life to ensure it remains effective over extended periods.


 Previous Story
Previous Story

 Latest articles
Latest articles 

Leave a Comment
Cancel